Diagnosis of nascent fibrosis: Interest of marking the relaxed form of Fibronectin fibres
Adama Sidibé1,2,3,* 
1-Editor-in-Chief of Cell Biology, Rviews Press, 13010, Marseille, France
2-Fibrocure – Research & Therapeutics, 1211, Geneva, Switzerland
3-Present research site address: c/o grp Wehrle-Haller, Faculty of Medicine, University of Geneva, 1211, Geneva, Switzerland
*Correspondence: asidibe@rviews.org
Abstract
Fibrosis is a pathological and irreversible state of excessively synthesized extracellular matrix fibres induced by abnormal tissue scarring. Effective diagnostic tools for early management of patients with fibrosis are missing. Here, I discuss the recent advances in the quantification of Fibronectin fibres and the interest of novel biologics for diagnosis.
Keywords
idiopathic pulmonary fibrosis; kidney fibrosis; PET/CT; biomarker; relaxed fibronectin; collagen; diagnosis; cancer
Tissue repair and fibrosis
Multiple circumstances including inflammation and diseases lead to tissue damages that need repair. The recurrent and exacerbated repair can lead to uncontrolled and irreversible scarring of the tissue with an excessive accumulation of extracellular matrix (ECM) proteins including the glycoproteins Fibronectin and Collagens1. Theoretically, all players in normal tissue repair are potential contributors to fibrosis in uncontrolled pathological conditions. Several players within the injured tissue may contribute and include the damaged cells that need clearance, the inflammatory immune cells that are recruited to the wound site releasing the necessary cell activation factors, the endothelial cells that allow immune cell recruitment and contribute to ECM synthesis and wound healing, and fibroblasts that are activated to proliferate and synthesize ECM fibres to provide the required mechanical strength to the repaired tissue2–4. However, when recurrent and exacerbated, the functions of those players become uncontrolled leading to fibrosis, an excessive accumulation of ECM proteins and irreversible scarring of the tissue5. Fibrotic scarring is deleterious for normal functioning of the tissue and alters normal cell survival, nutrient delivery as well as gas (oxygen and carbon dioxide) exchanges resulting in cell demises and forming regions made of only extracellular matrix components6,7. Fibrosis can occur in all organs but is often associated to sites of chronic inflammatory diseases and cancers8–13. Today, fibrosis is uncurable, but some clinical solutions are proposed to slow down its evolution14,15. If not managed early and properly, fibrosis may lead to complete unfunctional state of the tissue, resulting in organ failure and death16. There is an urgent need of diagnostic tools and biomarkers of nascent fibrosis that can allow an effective clinical management of the different forms of fibrosis. Developing efficient approaches for diagnosis, prognosis and therapy of fibrosis depends on a better understanding of the key molecular and pathogenetic mechanisms that support the synthesis of extracellular matrix proteins during normal as well as pathologic inflammation and wound healing (Fig. 1).
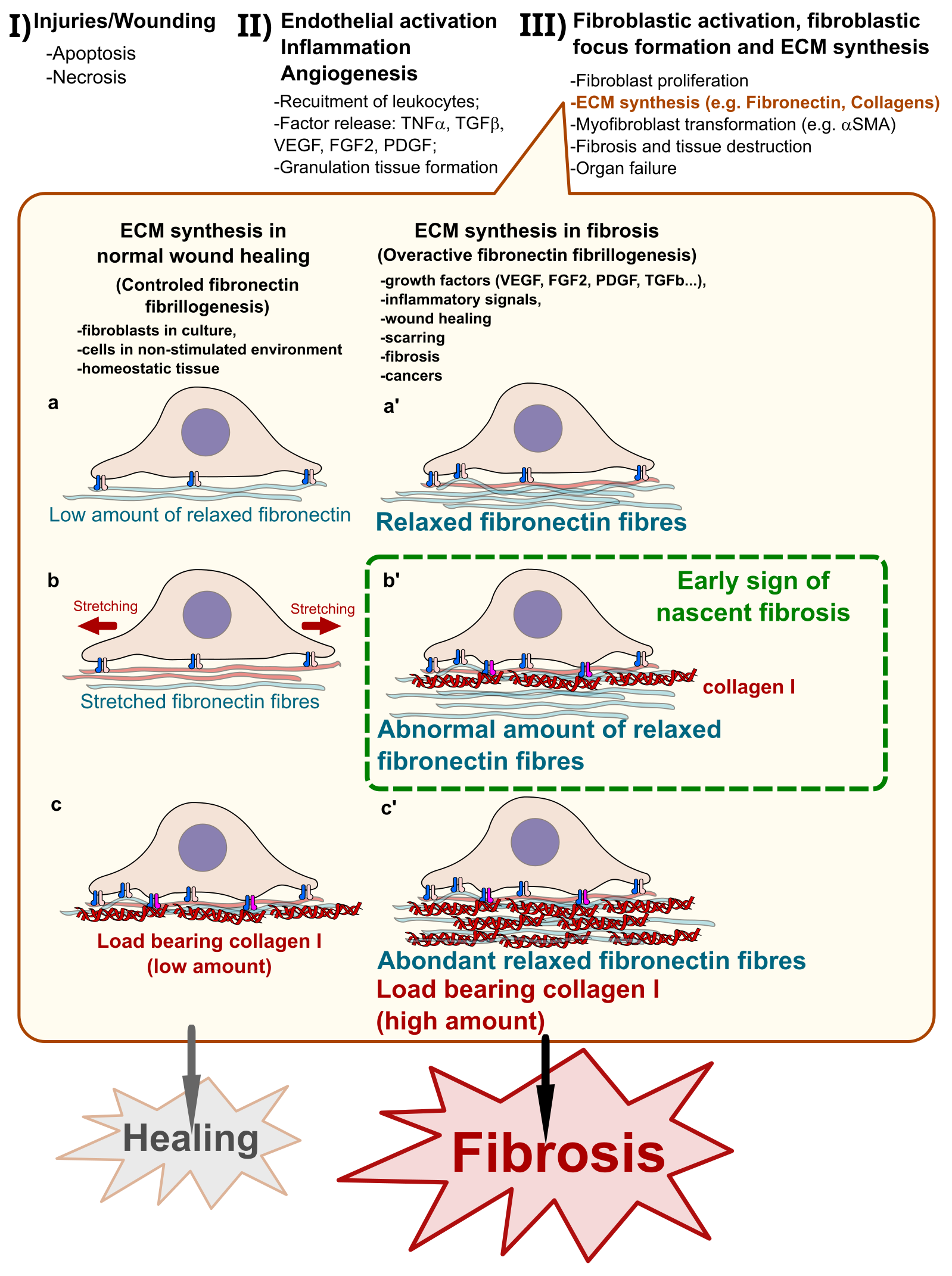
Figure 1: Pathogenesis of fibrosis and early diagnosis opportunity
I) Internal or external agents induce cell death by necrosis or apoptosis leading to tissue injuries/wounds that need to be repaired. II) Activation of endothelial cells initiates inflammation and induces the recruitment of immune cells that release large amount of growth and angiogenic factors such as VEGF, FGF2, TGFβ and PDGF that in turn trigger the granulation tissue formation for wound healing; III) wound closure and fibroblast activation, which proliferate, form fibroblast foci, synthesize ECM and transform into myofibroblasts in regions of high mechanical strain and high expression of Collagen. The ECM synthesis step of the pathogenesis offers an opportunity of detecting cases of nascent fibrosis. In normal wound healing process (a), fibroblasts synthesize Fibronectin fibres in a controlled fashion by making a relatively low amount of relaxed Fibronectin fibres, which are for most mechanically stretched by the cells (b) and the small remaining Fibronectin fibres are stabilized by low amount Collagen I fibres (c) allowing a normal healing of the wound. In pathological pre-fibrotic conditions, (a’ and b’) the exacerbated formation of the relaxed form of Fibronectin fibres induces the synthesis of abnormally high amount of Collagen I to bear the overload (c’). Probes such as adhesins-derivatives capable of specifically recognize the relaxed form of Fibronectin fibres provide an opportunity for tracking regions of nascent fibrosis and following the fibrotic activity in several chronic diseases.
Fibrosis: an issue of ECM synthesis
Our understanding of the pathogenesis of fibrosis has outstandingly advanced. From an inflammation-based conception, it is becoming clear that the primary cells involved in the excessive synthesis of ECM proteins such as myofibroblasts and endothelial cells deserve particular attention for the development of diagnostic and therapeutic tools2,5,17,18. In fact, inflammation is an important contributor to the fibrotic process. Inflammatory cells are recruited to sites of wounds provoked by cell apoptosis and/or necrosis induced by internal or external agents. The recruited immune cells participate in the clearance of tissue debris and release crucial cytokines and factors such as the basic fibroblast growth factor (bFGF or FGF2), the transforming growth factor beta (TGFβ), vascular endothelial growth factor (VEGF)-A or the platelet-derived growth factor (PDGF) that lead to the proliferation and transformation of fibroblasts into myofibroblasts19–24. Myofibroblasts that are characterized by the expression of alpha-smooth muscle actin (αSMA), are high mechanical tension developing mesenchymal cells that deposit ECM proteins including Fibronectin and Collagen fibres to regions of myofibroblastic foci5,18,25,26. Although the growth factors have important implications for the proliferation and transformation of myofibroblasts, the manner these cells interact with their environment as well as how the deposited matrix fibres support the applied mechanical load are particularly critical for a sustained de novo matrix deposition4,17. The current advances in our understanding of the myofibroblast transformation process as well as the mechanisms of ECM protein fibrillogenesis support the idea that fibrosis is primarily an issue of ECM synthesis and how mesenchymal cells feel the need of depositing high load-supporting matrix fibres such as Collagens.
Focus on ECM proteins for diagnosis
Current diagnosis
Fibrosis is currently diagnosed by multidisciplinary methods including histopathological features with the appearance of areas of cell demises with altered tissue architecture by excessive bundles of ECM fibres on tissue biopsies in most disease contexts. In some pathological conditions such as the idiopathic pulmonary fibrosis (IPF), the combination of symptoms, functional analysis by spirometry, endurance test such as six-minute walk test and imaging by high resolution computed tomography (HRCT) allows physicians to also appreciate the degree of usual interstitial pneumonia (UIP) as well as the lung functional alteration27,28. In cancer biopsies, the appearance of fibrosis is common and was associated with poor prognosis and the disease resistance to therapy including immunotherapy and chemotherapy10–12,29–32. However, no validated biomarker of fibrosis of any organs or tissue type was approved by the American Food and Drug Administration (FDA) for clinical use. In the example of IPF patients, several molecules found highly expressed were proposed to serve as biomarker of the disease and they include metalloproteinases (MMP) such as MMP1 and MMP7, the tissue inhibitors of MMPs such as TIMP-1, the surfactant proteins A and D (SPA and SPD), Krebs von den Lungen-6, Galectin-3, S100A12, proCollagen III N-terminal peptide, microRNAs and periostin33,34. None of these outlined putative biomarkers could serve as diagnostic tools due to the lack of specificity.
Fibronectin and its structural organization for early-stage fibrosis diagnosis
The hierarchical assembly and organization of ECM proteins is critical for normal development, wound healing but also for fibrotic scarring in fibrosis and cancers35–43. A better understanding of the hierarchical composition and assembly of ECM might provide a unique opportunity for developing the most relevant diagnostic tools for an early management of patients. In fact, in both normal and pathological processes, the active assembly of Fibronectin fibres is required to establish the needed scaffold for efficient Collagen I deposition in tissue interstitia44–47. Indeed, although the monomeric Collagen I can polymerize in vitro through entropy, its assembly in vivo require the Fibronectin scaffolding through a cell-mediated active fibrillogenesis48. Collagen-Fibronectin interactions through Fibronectin’s gelatin-binding domain is required for the initial deposition of Collagen fibres44. The binding of Collagen I to Fibronectin occurs through sites at the N-terminus of Fibronectin and encompass several modules including FnI6, FnII1-2 and FnI7-9. Interfering with Fibronectin expression or inhibiting the interaction of Collagen I with the Fibronectin scaffold were both found to block Collagen deposition in cell culture and preclinical experimental model in mouse49–52. Diagnostic methods based on monitoring the active fibrillogenesis of de novo Fibronectin fibres are relevant and might be interesting for detecting early-stage fibrosis or abnormal pre-fibrotic regions in organs.
Recent findings showed that the mechanical strain applied to the assembled Fibronectin scaffolds as well as the resulting secondary structure of the protein N-terminal region are determinant for Collagen I interaction4,53. Precisely, only assembled Fibronectin fibrils in relaxed form and under low mechanical strain can bind to Collagen I and lead to Collagen fibre assembly4. Thus, monitoring abnormal quantity of the relaxed form of Fibronectin fibres might provide with the most adequate method of diagnosing pre-fibrotic states as well as the early-stage fibrosis.
Bacterial adhesins target Fibronectin N-terminus
Adhesins from several bacterial strains including but not limited to Staphylococcus aureus, Streptococcus pyogenes, Streptococcus dysgalactiae, Streptococcus equisimilis, Borrelia burgdorferi and many others interact with Fibronectin fibres at the N-terminus and participate in the pathogenic infection of host cells54–56. Precisely for example, the modules FnI1-5 of Fibronectin N-terminus are recognized by motif repeats of the Fibronectin-binding proteins (FnBPs) of S. aureus (FnBPA1-11) and S. pyogenes (SfbI1-5)57. Most adhesins-derived proteins bind sites encompassing different FnI modules of Fibronectin with high affinity by forming anti-parallel tandem β-zippers58. Advances in this field showed that FnBPs can be used to distinguish between relaxed (under low strain) and stretched (under high strain) Fibronectin fibres in cells and at the tissue level59. Recently, the FnBB-4 B3 and FnBP5 from adhesins of respectively S. dysgalactiae and S. aureus were found incapable of binding stretched Fibronectin fibres due to structural mismatches60. In a proof-of-concept study, FnBP5 was found to bind relaxed Fibronectin in tumour sections, and colocalize with the myofibroblast marker αSMA as well as Collagen bundles of deposited Collagen revealed by the second harmonic generation signals59. Notably, in the same study, radiolabeled FnBP5 showed strong signals in prostate cancer (PC-3) xenografts in the animal analysis by positron emission tomography (PET). Adhesins-derived probes hold promising features for serving as diagnosis tools for the management of early-stage fibrosis in patients.
Probing Fibronectin revealed nascent fibrosis in experimental IPF
The functional upstream domain (FUD) of the S. pyogenes SfbI-containing adhesin was established to bind the site covering the N-terminus and gelatin-binding site of Fibronectin61. The FUD peptide showed inhibitory activity on Fibronectin fibrillogenesis as well as antifibrotic capacity in preclinical studies in mouse52. In addition, the FUD peptide was modified by adjunction of specific polyethylene glycol (PEG) moieties improving its pharmacokinetic properties in vivo62–64. Thereby, PEG-FUD was shown to target the deposition of Fibronectin and Collagen fibres in the bleomycin-induced murine pulmonary fibrosis model in vivo65.
Interestingly, in a recent study reported by Lee et al, the interest of the PEG-FUD was extended for use as a diagnostic tool for tracking nascent fibrosis in preclinical model of IPF65. Indeed, Lee et al brought strong proof-of-concept evidence that 64Cu radiolabeled PEG-FUD allows the detection of early fibrosis by PET/CT imaging. First by immunofluorescence, the authors showed in bleomycin-induced IPF that PEG-FUD strongly stained area of lung tissue with cell demise and high ECM synthesis as determined by anti-Fibronectin staining although presenting low coloration by Masson’s trichrome stain. The authors have shown the specificity of PEG-FUD by using a non-PEGylated FUD peptide as competitor, which substantially reduced the tissue staining. Interestingly, a mutated form of PEG-FUD (PEG-mFUD) with seven amino acid deletion, incapable of strongly interacting with Fibronectin62, was unable to stain the nascent fibrotic foci in lung. This was particularly an important demonstration as it showed how this particular PEGylation did not interfere with the FUD peptide binding to the exposed Fibronectin N-terminus.
Lee et al then developed a 64Cu-PEG-FUD to study the spatial distribution of early fibrotic regions in mouse treated by bleomycin. The ex vivo analysis of organs showed that lung slices presented strong signals of 64Cu-PEG-FUD compared to 64Cu-PEG-mFUD as early as eleven days post-treatment by bleomycin. The specific staining of lung slices by 64Cu-PEG-FUD was also shown in the decellularized tissue with similar results demonstrating that the insoluble extracellular fibres were the main target of the radiolabeled PEG-FUD consistent with previous reports. In PET/CT imaging of animals after three days of bleomycin treatment, 64Cu-PEG-FUD showed higher signal in lungs compared to 64Cu-PEG-mFUD. Notably, the radiodensity, a micro(µ)-CT characteristics of fibrotic tissue were not present at three days post-bleomycin treatment. Consistently, eleven days post-bleomycin treatment, the signal of 64Cu-PEG-FUD correlated with the µCT radiodensity demonstrating a correlation of the probe signal with the fibrotic activity in bleomycin-induced IPF model. Overall, Lee et al made the proof-of-concept and have demonstrated that the probes based on the radiolabeled 64Cu-PEG-FUD can be used to diagnose sites of nascent fibrosis by PET/CT imaging and that radiolabeled PEG-FUD might be a valuable tool for use in clinical practice for the follow up of patients with fibrosis including IPF.
One of the limitations of the use of 64Cu-PEG-FUD in PET/CT is the accumulation of the radio-signal in liver, an organ that produce large amount of Fibronectin. The heart and kidneys also presented high accumulation of the 64Cu-PEG-FUD probe early after the intravenous injection of the tracer. The heart signal might be due to blood circulation of the probe whereas the signal in kidney was likely caused by the elimination process as both organs showed similar signals with the saline-treated mice or with the 64Cu-PEG-mFUD mutant control in bleomycin-treated animals. A method that the authors proposed to mitigate the non-specific background signals of 64Cu-PEG-FUD probe in liver was to simultaneously inject an empirical amount of unlabeled PEG-FUD. Other parameters such as the latency between tracer injection and PET/CT imaging could also be interesting for optimizing the specific detection of pre-fibrotic or early fibrotic areas in organs. In the perspective, it would be of interest to study the correlation between the PET signal of 64Cu-PEG-FUD and the radiodensity of CT in more advanced models of fibrosis in mouse. The accumulating data suggests that it is time to start clinical trials in patients with advanced fibrosis in order to evaluate whether the PEGylated FUD and its derivatives can actually help clinician to analyze the fibrotic activity.
The FUD peptide as well as other FnBP motives derived from bacterial adhesins target the structured N-terminus of Fibronectin fibres. Strong evidence showed that under high mechanical strain, the stretched Fibronectin losses the second structure of its N-terminus preventing the bacterial adhesin binding to the fibres60. In addition, only the relaxed Fibronectin fibres that are under low mechanical strain require and allow Collagen fibre synthesis to bear the overload. This suggests that developing adhesins-derived sensors that specifically and efficiently probe the relaxed Fibronectin fibres is crucial for tracking nascent fibrotic regions of the tissue. Tracking the relaxed Fibronectin fibres in suspicious tissues may be more specific to pre-fibrosis or nascent fibrosis than broadly quantifying Fibronectin. The adhesins-derived sensors have the advantage to be small and recognize higher order organizational state when compared to antibodies, which can be affected by epitope hindrance in fibres in addition to their characteristic low penetration in tissue. New generation of adhesins-derived sensors are under development to improve their sensitivity and stability for efficiently tracking the relaxed or pro-fibrotic fibres of Fibronectin. This will allow their use in clinical routine to improve the management of patients susceptible of suffering from different fibrotic diseases including cancers, IPF, liver and kidney fibrosis.
Declaration of interests
Adama Sidibé is the Editor-In-Chief of Cell Reviews, Cell Biology and Cell Methods, three sister journals of Rviews Press, Marseille, France.
Adama Sidibé is the founder of Rviews Press (https://www.rviews.org) and Fibrocure-Research & Therapeutics (https://www.fibrocure.ch).
Declaration concerning generative AI use
The author declares that no generative artificial intelligence (AI) tools were used to make this manuscript.
Citing the article
Sidibé, A. (2024). Diagnosis of nascent fibrosis: Interest of marking the relaxed form of Fibronectin fibres. Cell Biology 1, 29-39. https://doi.org/10.70296/cb-16csqmvns8.
References
1. Wynn, T.A., and Ramalingam, T.R. (2012). Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18, 1028–1040. https://doi.org/10.1038/nm.2807.
2. Wynn, T.A. (2008). Cellular and molecular mechanisms of fibrosis. Journal of Pathology 214, 199–210. https://doi.org/10.1002/path.2277.
3. Henderson, N.C., Rieder, F., and Wynn, T.A. (2020). Fibrosis: from mechanisms to medicines. Nature 587, 555–566. https://doi.org/10.1038/s41586-020-2938-9.
4. Kubow, K.E., Vukmirovic, R., Zhe, L., Klotzsch, E., Smith, M.L., Gourdon, D., Luna, S., and Vogel, V. (2015). Mechanical forces regulate the interactions of fibronectin and collagen i in extracellular matrix. Nature Communications 6, 1–11. https://doi.org/10.1038/ncomms9026.
5. Gabbiani, G. (2003). The myofibroblast in wound healing and fibrocontractive diseases. Journal of Pathology 200, 500–503. https://doi.org/10.1002/path.1427.
6. Sidibe, A., Polena, H., Pernet-Gallay, K., Razanajatovo, J., Mannic, T., Chaumontel, N., Bama, S., Marechal, I., Huber, P., Gulino-Debrac, D., et al. (2014). VE-cadherin Y685F knock-in mouse is sensitive to vascular permeability in recurrent angiogenic organs. Am J Physiol Heart Circ Physiol 307, H455-63. https://doi.org/10.1152/ajpheart.00774.2013.
7. Luo, L., Zhang, W., You, S., Cui, X., Tu, H., Yi, Q., Wu, J., and Liu, O. (2024). The role of epithelial cells in fibrosis: Mechanisms and treatment. Pharmacological Research 202, 107144. https://doi.org/10.1016/j.phrs.2024.107144.
8. Selman, M., King, T.E., and Pardo, A. (2001). Idiopathic Pulmonary Fibrosis: Prevailing and Evolving Hypotheses about Its Pathogenesis and Implications for Therapy. Ann Intern Med 134, 136. https://doi.org/10.7326/0003-4819-134-2-200101160-00015.
9. King, T.E., Pardo, A., and Selman, M. (2011). Idiopathic pulmonary fibrosis. The Lancet 378, 1949–1961. https://doi.org/10.1016/S0140-6736(11)60052-4.
10. Abyaneh, H.S., Regenold, M., McKee, T.D., Allen, C., and Gauthier, M.A. (2020). Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics 10, 1960–1980. https://doi.org/10.7150/thno.39995.
11. Teisseire, M., Giuliano, S., and Pagès, G. (2024). Combination of Anti-Angiogenics and Immunotherapies in Renal Cell Carcinoma Show Their Limits: Targeting Fibrosis to Break through the Glass Ceiling? Biomedicines 12, 385. https://doi.org/10.3390/biomedicines12020385.
12. Watson, S.S., Zomer, A., Fournier, N., Lourenco, J., Quadroni, M., Chryplewicz, A., Nassiri, S., Aubel, P., Avanthay, S., Croci, D., et al. (2024). Fibrotic response to anti-CSF-1R therapy potentiates glioblastoma recurrence. Cancer Cell 42, 1507-1527.e11. https://doi.org/10.1016/j.ccell.2024.08.012.
13. Ambrosetti, D., Coutts, M., Paoli, C., Durand, M., Borchiellini, D., Montemagno, C., Rastoin, O., Borderie, A., Grepin, R., Rioux-Leclercq, N., et al. (2022). Cancer-associated fibroblasts in renal cell carcinoma: implication in prognosis and resistance to anti-angiogenic therapy. BJU International 129, 80–92. https://doi.org/10.1111/bju.15506.
14. Klinkhammer, B.M., Goldschmeding, R., Floege, J., and Boor, P. (2017). Treatment of Renal Fibrosis—Turning Challenges into Opportunities. Advances in Chronic Kidney Disease 24, 117–129. https://doi.org/10.1053/j.ackd.2016.11.002.
15. Glass, D.S., Grossfeld, D., Renna, H.A., Agarwala, P., Spiegler, P., DeLeon, J., and Reiss, A.B. (2022). Idiopathic pulmonary fibrosis: Current and future treatment. The Clinical Respiratory Journal 16, 84–96. https://doi.org/10.1111/crj.13466.
16. Hoyer, N., Prior, T.S., Bendstrup, E., Wilcke, T., and Shaker, S.B. (2019). Risk factors for diagnostic delay in idiopathic pulmonary fibrosis. Respiratory Research 20, 103. https://doi.org/10.1186/s12931-019-1076-0.
17. Tomasek, J.J., Gabbiani, G., Hinz, B., Chaponnier, C., and Brown, R.A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3, 349–363. https://doi.org/10.1038/nrm809.
18. Herrera, J., Henke, C.A., and Bitterman, P.B. (2018). Extracellular matrix as a driver of progressive fibrosis. Journal of Clinical Investigation 128, 45–53. https://doi.org/10.1172/JCI93557.
19. Meng, X.M., Nikolic-Paterson, D.J., and Lan, H.Y. (2016). TGF-beta: the master regulator of fibrosis. Nature Reviews Nephrology 12, 325–338. https://doi.org/10.1038/nrneph.2016.48.
20. Ramachandran, P., Dobie, R., Wilson-Kanamori, J.R., Dora, E.F., Henderson, B.E.P., Luu, N.T., Portman, J.R., Matchett, K.P., Brice, M., Marwick, J.A., et al. (2019). Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518. https://doi.org/10.1038/s41586-019-1631-3.
21. Chaudhary, N.I., Roth, G.J., Hilberg, F., Müller-Quernheim, J., Prasse, A., Zissel, G., Schnapp, A., and Park, J.E. (2007). Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. European Respiratory Journal 29, 976–985. https://doi.org/10.1183/09031936.00152106.
22. Kendall, R.T., and Feghali-Bostwick, C.A. (2014). Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 5. https://doi.org/10.3389/fphar.2014.00123.
23. Strutz, F., Zeisberg, M., Hemmerlein, B., Sattler, B., Hummel, K., Becker, V., and Müller, G.A. (2000). Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney International 57, 1521–1538. https://doi.org/10.1046/j.1523-1755.2000.00997.x.
24. Inoue, Y., King, T.E., Tinkle, S.S., Dockstader, K., and Newman, L.S. (1996). Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am J Pathol 149, 2037–2054. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1865345/.
25. Knittel, T., Kobold, D., Piscaglia, F., Saile, B., Neubauer, K., Mehde, M., Timpl, R., and Ramadori, G. (1999). Localization of liver myofibroblasts and hepatic stellate cells in normal and diseased rat livers: distinct roles of (myo-)fibroblast subpopulations in hepatic tissue repair. Histochemistry and Cell Biology 112, 387–401. https://doi.org/10.1007/s004180050421.
26. Herrera, J.A., Dingle, L., Montero, M.A., Venkateswaran, R.V., Blaikley, J.F., Lawless, C., and Schwartz, M.A. (2022). The UIP/IPF fibroblastic focus is a collagen biosynthesis factory embedded in a distinct extracellular matrix. JCI Insight 7, e156115. https://doi.org/10.1172/jci.insight.156115.
27. Raghu, G., Remy-Jardin, M., Myers, J.L., Richeldi, L., Ryerson, C.J., Lederer, D.J., Behr, J., Cottin, V., Danoff, S.K., Morell, F., et al. (2018). Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 198, e44–e68. https://doi.org/10.1164/rccm.201807-1255ST.
28. Wells, A.U. (2013). Managing diagnostic procedures in idiopathic pulmonary fibrosis. European Respiratory Review 22, 158–162. https://doi.org/10.1183/09059180.00001213.
29. Rhim, A.D., Oberstein, P.E., Thomas, D.H., Mirek, E.T., Palermo, C.F., Sastra, S.A., Dekleva, E.N., Saunders, T., Becerra, C.P., Tattersall, I.W., et al. (2014). Stromal Elements Act to Restrain, Rather Than Support, Pancreatic Ductal Adenocarcinoma. Cancer Cell 25, 735–747. https://doi.org/10.1016/j.ccr.2014.04.021.
30. Sherman, M.H., Yu, R.T., Engle, D.D., Ding, N., Atkins, A.R., Tiriac, H., Collisson, E.A., Connor, F., Van Dyke, T., Kozlov, S., et al. (2014). Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 159, 80–93. https://doi.org/10.1016/j.cell.2014.08.007.
31. Egeblad, M., Ewald, A.J., Askautrud, H.A., Truitt, M.L., Welm, B.E., Bainbridge, E., Peeters, G., Krummel, M.F., and Werb, Z. (2008). Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech 1, 155–167; discussion 165. https://doi.org/10.1242/dmm.000596.
32. Olive, K.P., Jacobetz, M.A., Davidson, C.J., Gopinathan, A., McIntyre, D., Honess, D., Madhu, B., Goldgraben, M.A., Caldwell, M.E., Allard, D., et al. (2009). Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science 324, 1457–1461. https://doi.org/10.1126/science.1171362.
33. Zheng, Z., Peng, F., and Zhou, Y. (2024). Biomarkers in idiopathic pulmonary fibrosis: Current insight and future direction. Chinese Medical Journal Pulmonary and Critical Care Medicine 2, 72–79. https://doi.org/10.1016/j.pccm.2024.04.003.
34. Ley, B., Brown, K.K., and Collard, H.R. (2014). Molecular biomarkers in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 307, L681–L691. https://doi.org/10.1152/ajplung.00014.2014.
35. Egeblad, M., Rasch, M.G., and Weaver, V.M. (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Current Opinion in Cell Biology 22, 697–706. https://doi.org/10.1016/j.ceb.2010.08.015.
36. Astrof, S., and Hynes, R.O. (2009). Fibronectins in vascular morphogenesis. Angiogenesis 12, 165–175. https://doi.org/10.1007/s10456-009-9136-6.
37. Carver, W., and Goldsmith, E.C. (2013). Regulation of Tissue Fibrosis by the Biomechanical Environment. BioMed Research International 2013, 1–10. https://doi.org/10.1155/2013/101979.
38. Rozario, T., and DeSimone, D.W. (2010). The extracellular matrix in development and morphogenesis: A dynamic view. Developmental Biology 341, 126–140. https://doi.org/10.1016/j.ydbio.2009.10.026.
39. Reinke, J.M., and Sorg, H. (2012). Wound Repair and Regeneration. Eur Surg Res 49, 35–43. https://doi.org/10.1159/000339613.
40. Miles, F.L., and Sikes, R.A. (2014). Insidious Changes in Stromal Matrix Fuel Cancer Progression. Molecular Cancer Research 12, 297–312. https://doi.org/10.1158/1541-7786.MCR-13-0535.
41. Schäfer, M., and Werner, S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol 9, 628–638. https://doi.org/10.1038/nrm2455.
42. Cukierman, E., and Bassi, D.E. (2010). Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Seminars in Cancer Biology 20, 139–145. https://doi.org/10.1016/j.semcancer.2010.04.004.
43. Midwood, K.S., Williams, L.V., and Schwarzbauer, J.E. (2004). Tissue repair and the dynamics of the extracellular matrix. The International Journal of Biochemistry & Cell Biology 36, 1031–1037. https://doi.org/10.1016/j.biocel.2003.12.003.
44. McDonald, J.A., Kelley, D.G., and Broekelmann, T.J. (1982). Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. The Journal of cell biology 92, 485–492. https://doi.org/10.1083/jcb.92.2.485.
45. Kadler, K.E., Hill, A., and Canty-Laird, E.G. (2008). Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Current Opinion in Cell Biology 20, 495–501. https://doi.org/10.1016/j.ceb.2008.06.008.
46. Dzamba, B.J., Wu, H., Jaenisch, R., and Peters, D.M. (1993). Fibronectin binding site in type I collagen regulates fibronectin fibril formation. The Journal of cell biology 121, 1165–1172. https://doi.org/10.1083/jcb.121.5.1165.
47. McDonald, J.A., Quade, B.J., Broekelmann, T.J., LaChance, R., Forsman, K., Hasegawa, E., and Akiyama, S. (1987). Fibronectin’s cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. Journal of Biological Chemistry 262, 2957–2967. https://doi.org/10.1016/S0021-9258(18)61453-X.
48. Li, S., Van Den Diepstraten, C., D’Souza, S.J., Chan, B.M.C., and Pickering, J.G. (2003). Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. Am J Pathol 163, 1045–1056. https://doi.org/10.1016/s0002-9440(10)63464-5.
49. Filla, M.S., Dimeo, K.D., Tong, T., and Peters, D.M. (2017). Disruption of fibronectin matrix affects type IV collagen, fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Experimental Eye Research 165, 7–19. https://doi.org/10.1016/j.exer.2017.08.017.
50. Sottile, J., Shi, F., Rublyevska, I., Chiang, H.-Y., Lust, J., and Chandler, J. (2007). Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. American Journal of Physiology-Cell Physiology 293, C1934–C1946. https://doi.org/10.1152/ajpcell.00130.2007.
51. Sottile, J., and Hocking, D.C. (2002). Fibronectin Polymerization Regulates the Composition and Stability of Extracellular Matrix Fibrils and Cell-Matrix Adhesions. MBoC 13, 3546–3559. https://doi.org/10.1091/mbc.e02-01-0048.
52. Altrock, E., Sens, C., Wuerfel, C., Vasel, M., Kawelke, N., Dooley, S., Sottile, J., and Nakchbandi, I.A. (2015). Inhibition of fibronectin deposition improves experimental liver fibrosis. Journal of Hepatology 62, 625–633. https://doi.org/10.1016/j.jhep.2014.06.010.
53. Antia, M., Baneyx, G., Kubow, K.E., and Vogel, V. (2008). Fibronectin in aging extracellular matrix fibrils is progressively unfolded by cells and elicits an enhanced rigidity response. Faraday Discuss. 139, 229. https://doi.org/10.1039/b718714a.
54. Sottile, J., Schwarzbauer, J., Selegue, J., and Mosher, D.F. (1991). Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. Journal of Biological Chemistry 266, 12840–12843. https://doi.org/10.1016/S0021-9258(18)98769-7.
55. Peake, P., Gooley, A., and Britton, W.J. (1993). Mechanism of interaction of the 85B secreted protein of Mycobacterium bovis with fibronectin. Infect Immun 61, 4828–4834. https://doi.org/10.1128/iai.61.11.4828-4834.1993.
56. Lindmark, H., and Guss, B. (1999). SFS, a Novel Fibronectin-Binding Protein from Streptococcus equi , Inhibits the Binding between Fibronectin and Collagen. Infect Immun 67, 2383–2388. https://doi.org/10.1128/IAI.67.5.2383-2388.1999.
57. Joh, D., Wann, E.R., Kreikemeyer, B., Speziale, P., and Höök, M. (1999). Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biology 18, 211–223. https://doi.org/10.1016/S0945-053X(99)00025-6.
58. Schwarz-Linek, U., Gurusiddappa, S., Kim, J.H., Pilka, E.S., Briggs, J.A.G., Gough, T.S., Campbell, I.D., and Potts, J.R. (2003). Pathogenic bacteria attach to human fibronectin through a tandem b-zipper. Nature 423. https://doi.org/10.1038/nature01589.
59. Arnoldini, S., Moscaroli, A., Chabria, M., Hilbert, M., Hertig, S., Schibli, R., Béhé, M., and Vogel, V. (2017). Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. Nat Commun 8, 1793. https://doi.org/10.1038/s41467-017-01846-0.
60. Chabria, M., Hertig, S., Smith, M.L., and Vogel, V. (2010). Stretching fibronectin fibres disrupts binding of bacterial adhesins by physically destroying an epitope. Nat Commun 1, 135. https://doi.org/10.1038/ncomms1135.
61. Tomasini-Johansson, B.R., Kaufman, N.R., Ensenberger, M.G., Ozeri, V., Hanski, E., and Mosher, D.F. (2001). A 49-Residue Peptide from Adhesin F1 of Streptococcus pyogenes Inhibits Fibronectin Matrix Assembly. Journal of Biological Chemistry 276, 23430–23439. https://doi.org/10.1074/jbc.M103467200.
62. Tomasini-Johansson, B.R., Zbyszynski, P.W., Toraason, I., Peters, D.M., and Kwon, G.S. (2018). PEGylated pUR4/FUD peptide inhibitor of fibronectin fibrillogenesis decreases fibrosis in murine Unilateral Ureteral Obstruction model of kidney disease. PLOS ONE 13, e0205360. https://doi.org/10.1371/journal.pone.0205360.
63. Zbyszynski, P., Tomasini-Johansson, B.R., Peters, D.M., and Kwon, G.S. (2018). Characterization of the PEGylated Functional Upstream Domain Peptide (PEG-FUD): a Potent Fibronectin Assembly Inhibitor with Potential as an Anti-Fibrotic Therapeutic. Pharm Res 35, 126. https://doi.org/10.1007/s11095-018-2412-7.
64. Zbyszynski, P., Toraason, I., Repp, L., and Kwon, G.S. (2019). Probing the subcutaneous absorption of a PEGylated FUD peptide nanomedicine via in vivo fluorescence imaging. Nano Convergence 6, 22. https://doi.org/10.1186/s40580-019-0192-3.
65. Lee, H.J., Bernau, K., Harr, T.J., Rosenkrans, Z.T., Kessler, G.A., Stott, K., Oler, A.T., Rahar, B., Zhu, T., Medina-Guevara, Y., et al. (2024). [64Cu]Cu-PEG-FUD peptide for noninvasive and sensitive detection of murine pulmonary fibrosis. Science Advances 10, eadj1444. https://doi.org/10.1126/sciadv.adj1444.
Notice
Publisher : Rviews Press, 181 rue Pierre DOIZE, 13010, Marseille, France (https://www.rviews.org)
Journal : Cell Biology (Marseille, France)
Producer: Dr Adama Sidibé
Editor: Dr Adama Sidibé
Director of publication: Dr Adama Sidibé
Contact: asidibe@rviews.org
The articles published in Cell Biology are distributed under the Creative Commons Attribution 4.0 International (CC BY)
Copyright © 2024 The author, Rviews Press Marseille, France. All right reserved including those for text, images, AI training and AI-like technologies
No responsibility is assumed by the publisher for any injury to persons or problem of products liability or otherwise, or from any use of any methods, products, instructions or as simple as ideas contained in this material.
Due to the rapid progress in the medical sciences- and related fields, independent analysis and verification of the referred materials, products or articles should be done. Independent diagnoses and drug dosages should be made.
|

